Winrevair: Potential Blockbuster in PAH
Key Takeaways
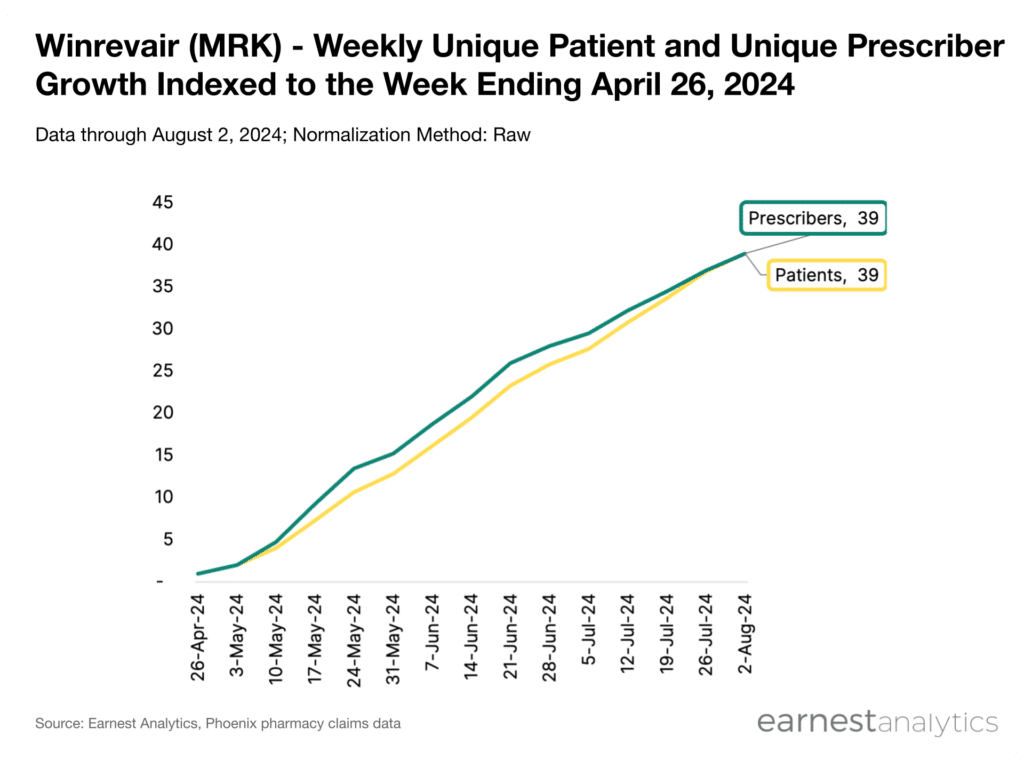
- Earnest’s Phoenix pharmacy claims data indicates continued strength in Winrevair’s commercial launch trajectory, with indexed cumulative unique patient and unique prescriber volume growing roughly 40x relative to its first week following launch
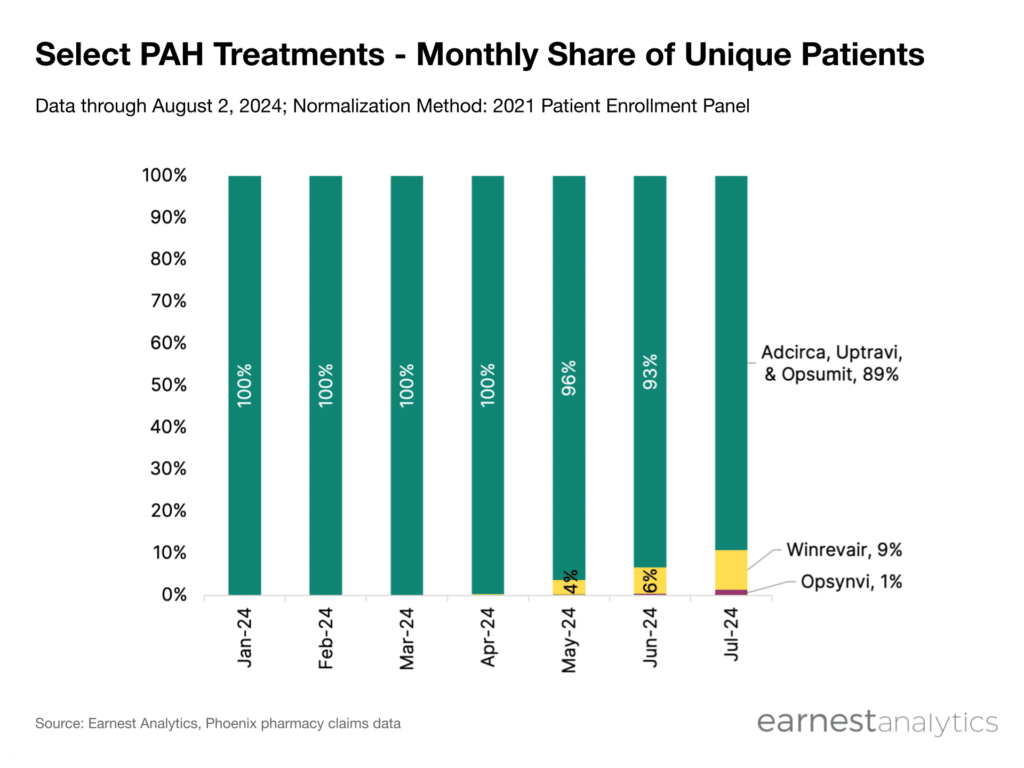
- Comparing Winrevair’s initial patient pool against key competitors Uptravi, Opsumit, and Adcirca suggests significant growth opportunities for Winrevair’s in its addressable market and a lead over the newest combination therapy Opsynvi
Winrevair (MRK) was approved by the FDA on March 26, 2024 and is a breakthrough, first-in-class therapy targeting the underlying cause of pulmonary arterial hypertension (PAH). PAH is a vascular disease of the lungs that is chronic, progressive, and ultimately fatal with a survival rate of approximately 50% five years post-diagnosis. Winrevair offers a new therapeutic option that could not only improve disease severity but also significantly improve quality of life for patients.
Earnest’s Phoenix pharmacy claims data indicates strong initial launch dynamics for Winrevair with continued weekly growth in its patient pool and prescriber base. Indexing to the first week of Winrevair data (week of April 20, 2024) cumulative unique patients and unique prescribers have grown roughly 40x as of August 2, 2024.
Contact Sales for details.
The treatment paradigm for PAH is crowded with several oral and subcutaneous options available on the market, and patients often take a combination of therapies to help manage their symptoms. Earnest pharmacy claims data indicates that >70% of patients starting on Winrevair have taken one of the key competitors Uptravi (JNJ), Opsumit (JNJ) and Adcirca (UTHR) within 1-year prior to starting Winrevair. Comparing the number of monthly unique patients on Winrevair against patients on any one or combination of those key competitors suggests significant growth opportunities for Winrevair over the course of its launch. Opsynvi (JNJ), which also launched in March 2024 and combines the active ingredients in Opsumit and Adcirca, appears to lag relative to Winrevair when comparing the initial number of patients on therapy.
Does not add to 100 due to rounding. Contact Sales for details.
In addition to being a life changing therapy for patients, Winrevair is expected to be a key driver of continued growth for Merck given that its cornerstone drug Keytruda, which made up 41.6% of their sales in 2023, faces a patent cliff in 2028. Continue tracking Winrevair’s real-world adoption and utilization trends with Earnest pharmacy claims data.
Request information on pharmacy claims data