Camzyos: early claims data trends pointing to strong therapy adherence

Key Takeaways
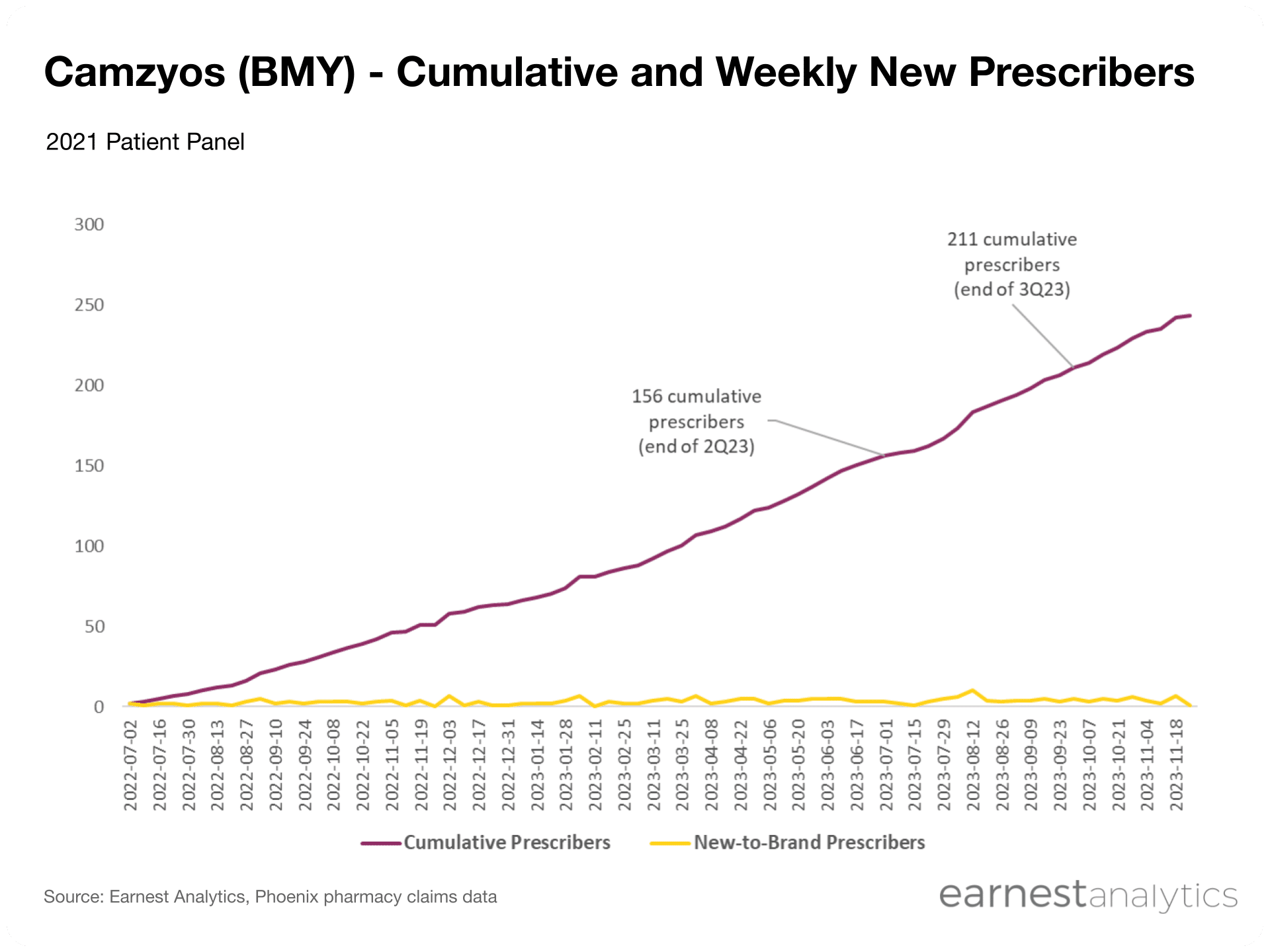
- Camzyos’ prescriber base is growing steadily despite its upfront REMS certification requirements, with cumulative prescribers growing by ~35% from end of 2Q2023 to end of 3Q2023
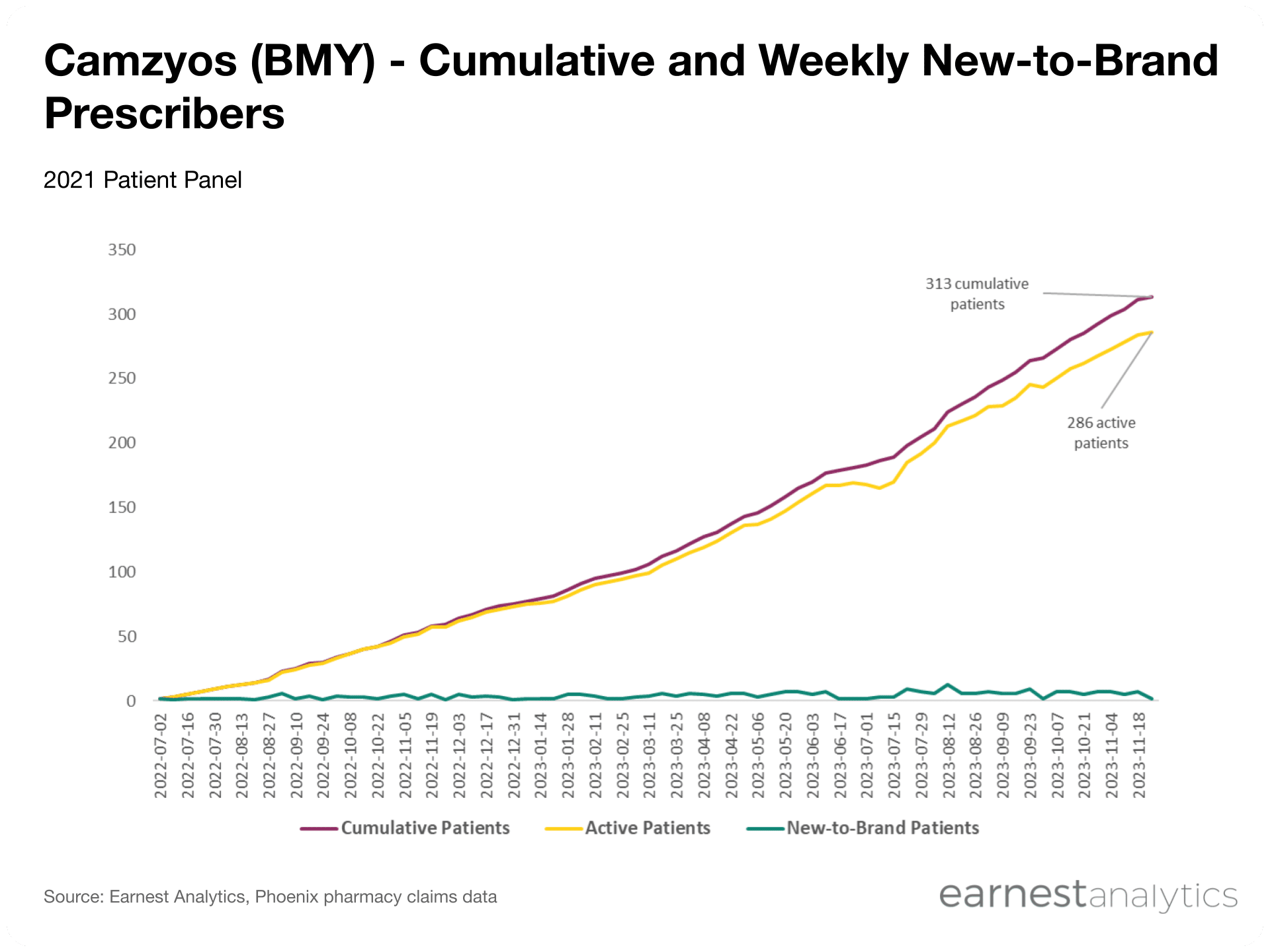
- Early patient retention trends indicate exceptional Camzyos adherence; from launch through mid-November 2023, roughly ~91% of cumulative commercial drug patients remain active on therapy
Camzyos (BMY) was approved by the FDA in April 2022 and is a first-in-class myosin inhibitor for the treatment of adults with symptomatic New York Heart Association class I-II obstructive hypertrophic cardiomyopathy (oHCM).
Despite REMS certification hurdles, Camzyos’ prescriber base has grown steadily since launch
Contact Sales for more details.
Camzyos carries a black box warning for heart failure due to systolic dysfunction and, as such, the FDA requires that prescribers are certified through BMY’s Risk Evaluation and Mitigation Strategy (REMS) program. Despite this initial barrier to entry, Earnest data shows consistent and sustained growth in unique prescribers. From the end of 2Q2023 to the end of 3Q2023, Camzyos cumulative prescribers grew by ~35%.
Early retention trends indicate Camzyos commercial drug patients are “sticky”
Contact Sales for more details.
In clinical trials, Camzyos significantly decreased obstruction of blood flow within the heart and improved overall symptom burden – many Camzyos patients reported improvements in exercise capacity and less shortness of breath. BMY management has commented that patients who start Camzyos “never want to come off” due to the benefits they see with treatment. Our Phoenix prescription data corroborates management enthusiasm and indicates that, from launch through mid-November 2023, roughly ~91% of cumulative commercial drug patients have continued to stay on Camzyos.
Camzyos is a cornerstone for BMY’s cardiovascular portfolio considering top-selling Eliquis will eventually face loss of exclusivity later this decade. Despite REMS program restrictions, recent prescriber growth and early retention trends for commercial drug patients bode well for BMY. Continue tracking Camzyos’ real-world adoption and utilization trends with Earnest data.